01
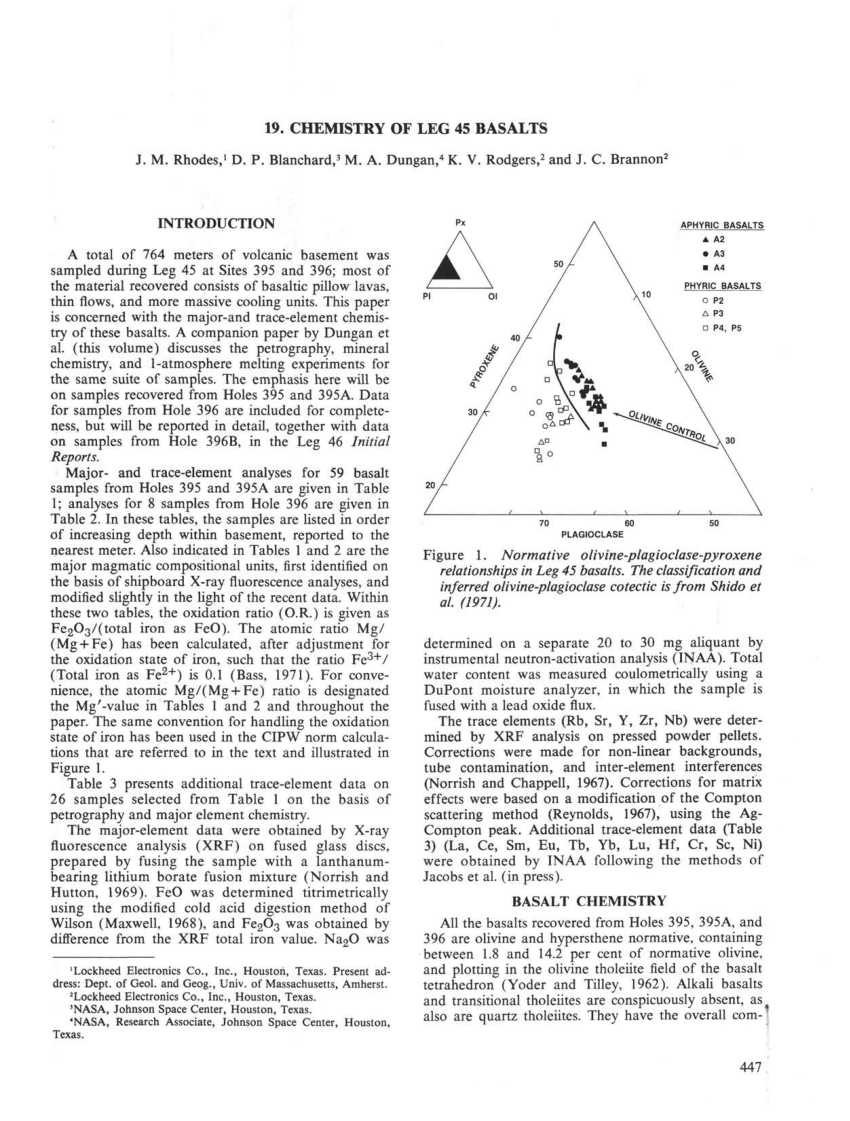
янв
Tkp 45 302 70 2009
Posted:adminTkp 45 3.02-240-2011 (02250) Tkp 45 3.02-6-2005 (02250) Tkp 45 3. (02250) Tkp 45 3.02-7-2005 (02250) Tkp 45 3.
Spinal cord injury (SCI) induces an immune response during which microglia, the resident immunocompetent cells of the central nervous system, become activated and migrate to the site of damage. Depending on their state of activation, microglia secrete neurotoxic or neurotrophic factors that influence the surrounding environment and have a detrimental or restorative effect following SCI, including causing or protecting bystander damage to nearby undamaged tissue.
Subsequent infiltration of macrophages contributes to the SCI outcome. 
Microglia/macrophages located within or proximal to the lesion produced neurotoxic factors, such as tumor necrosis factor alpha (TNF-α). These results suggest that microglia/macrophages within the epicenter at early time points post injury are neurotoxic, contributing to demyelination and axonal degeneration and that MIF/TKP could be used in combination with other therapies to promote functional recovery. Introduction Spinal cord injury (SCI) is a devastating trauma marked by the loss of sensory, motor and autonomic functions due to the degeneration of axons and glia. The initial insult to the central nervous system (CNS) causes necrosis of cells within the lesion epicenter, thereby inducing a cascade of biochemical and cellular events that lead to demyelination, degeneration of spared axons and inflammatory immune responses collectively referred to as secondary damage (; ). The necrosis triggers glial cell (e.g.
Astrocytes, oligodendrocyte precursor cells, and microglia) activation and recruitment to the site of injury. Glial cell activation and the subsequent gliotic scar formation are thought to drive the ensuing secondary damage.
In this setting, microglia, the resident CNS immune cells, release proinflammatory cytokines, reactive oxygen species and proteases that create bystander damage (; ). Chemokines produced by microglia, such as CCL2, CCL3, CXCL2/3 and CXCL10 are upregulated in minutes following injury (), recruit and activate peripheral polymorphonuclear leukocytes and monocytes which have been demonstrated to promote neurotoxicity (;;; ).
Model j ekonomiki kitaya prezentaciya. On the other hand, the phagocytic properties of microglia and macrophages ensure myelin and tissue debris removal following the initial insult, which is necessary for proper remyelination of axons and neurite outgrowth (). In subsequent phases of the immune reaction, peripheral inflammatory cells such as neutrophils, macrophages and T-lymphocytes enter the lesioned area and release a plethora of cytokines that mediate detrimental effects through the generation of free radicals which promote apoptosis ().
Popular Posts
Tkp 45 3.02-240-2011 (02250) Tkp 45 3.02-6-2005 (02250) Tkp 45 3. (02250) Tkp 45 3.02-7-2005 (02250) Tkp 45 3.

Spinal cord injury (SCI) induces an immune response during which microglia, the resident immunocompetent cells of the central nervous system, become activated and migrate to the site of damage. Depending on their state of activation, microglia secrete neurotoxic or neurotrophic factors that influence the surrounding environment and have a detrimental or restorative effect following SCI, including causing or protecting bystander damage to nearby undamaged tissue.
Subsequent infiltration of macrophages contributes to the SCI outcome. 
Microglia/macrophages located within or proximal to the lesion produced neurotoxic factors, such as tumor necrosis factor alpha (TNF-α). These results suggest that microglia/macrophages within the epicenter at early time points post injury are neurotoxic, contributing to demyelination and axonal degeneration and that MIF/TKP could be used in combination with other therapies to promote functional recovery. Introduction Spinal cord injury (SCI) is a devastating trauma marked by the loss of sensory, motor and autonomic functions due to the degeneration of axons and glia. The initial insult to the central nervous system (CNS) causes necrosis of cells within the lesion epicenter, thereby inducing a cascade of biochemical and cellular events that lead to demyelination, degeneration of spared axons and inflammatory immune responses collectively referred to as secondary damage (; ). The necrosis triggers glial cell (e.g.
Astrocytes, oligodendrocyte precursor cells, and microglia) activation and recruitment to the site of injury. Glial cell activation and the subsequent gliotic scar formation are thought to drive the ensuing secondary damage.
In this setting, microglia, the resident CNS immune cells, release proinflammatory cytokines, reactive oxygen species and proteases that create bystander damage (; ). Chemokines produced by microglia, such as CCL2, CCL3, CXCL2/3 and CXCL10 are upregulated in minutes following injury (), recruit and activate peripheral polymorphonuclear leukocytes and monocytes which have been demonstrated to promote neurotoxicity (;;; ).
Model j ekonomiki kitaya prezentaciya. On the other hand, the phagocytic properties of microglia and macrophages ensure myelin and tissue debris removal following the initial insult, which is necessary for proper remyelination of axons and neurite outgrowth (). In subsequent phases of the immune reaction, peripheral inflammatory cells such as neutrophils, macrophages and T-lymphocytes enter the lesioned area and release a plethora of cytokines that mediate detrimental effects through the generation of free radicals which promote apoptosis ().
...">Tkp 45 302 70 2009(01.01.2019)Tkp 45 3.02-240-2011 (02250) Tkp 45 3.02-6-2005 (02250) Tkp 45 3. (02250) Tkp 45 3.02-7-2005 (02250) Tkp 45 3.

Spinal cord injury (SCI) induces an immune response during which microglia, the resident immunocompetent cells of the central nervous system, become activated and migrate to the site of damage. Depending on their state of activation, microglia secrete neurotoxic or neurotrophic factors that influence the surrounding environment and have a detrimental or restorative effect following SCI, including causing or protecting bystander damage to nearby undamaged tissue.
Subsequent infiltration of macrophages contributes to the SCI outcome. 
Microglia/macrophages located within or proximal to the lesion produced neurotoxic factors, such as tumor necrosis factor alpha (TNF-α). These results suggest that microglia/macrophages within the epicenter at early time points post injury are neurotoxic, contributing to demyelination and axonal degeneration and that MIF/TKP could be used in combination with other therapies to promote functional recovery. Introduction Spinal cord injury (SCI) is a devastating trauma marked by the loss of sensory, motor and autonomic functions due to the degeneration of axons and glia. The initial insult to the central nervous system (CNS) causes necrosis of cells within the lesion epicenter, thereby inducing a cascade of biochemical and cellular events that lead to demyelination, degeneration of spared axons and inflammatory immune responses collectively referred to as secondary damage (; ). The necrosis triggers glial cell (e.g.
Astrocytes, oligodendrocyte precursor cells, and microglia) activation and recruitment to the site of injury. Glial cell activation and the subsequent gliotic scar formation are thought to drive the ensuing secondary damage.
In this setting, microglia, the resident CNS immune cells, release proinflammatory cytokines, reactive oxygen species and proteases that create bystander damage (; ). Chemokines produced by microglia, such as CCL2, CCL3, CXCL2/3 and CXCL10 are upregulated in minutes following injury (), recruit and activate peripheral polymorphonuclear leukocytes and monocytes which have been demonstrated to promote neurotoxicity (;;; ).
Model j ekonomiki kitaya prezentaciya. On the other hand, the phagocytic properties of microglia and macrophages ensure myelin and tissue debris removal following the initial insult, which is necessary for proper remyelination of axons and neurite outgrowth (). In subsequent phases of the immune reaction, peripheral inflammatory cells such as neutrophils, macrophages and T-lymphocytes enter the lesioned area and release a plethora of cytokines that mediate detrimental effects through the generation of free radicals which promote apoptosis ().
...">Tkp 45 302 70 2009(01.01.2019)